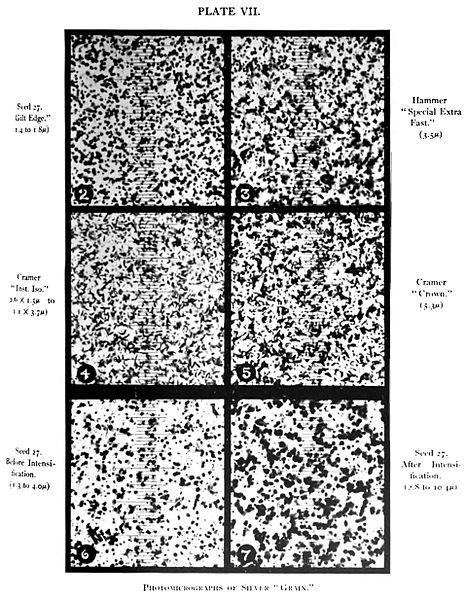
Figure 1 – Silver halide photographic grains, from the Wikicommons and in the public domain. Originally from Plate VII from Robert James Wallace, “The Silver ‘Grain’ in Photography” by Robert James Wallace, The Astrophysical Journal, Vol. XX, No. 2, Sept. 1904, pp. 113–122, Chicago.
Today, let’s continue with our discussion of the silver halide process. In our previous blog, we discussed the latent image, how it was made, and how you cannot see it. We discussed how a silver ion in the silver bromide, AgBr, grain, Ag+, gained an electron and became free silver, Ag. This process of gaining electrons in chemistry is called “reduction.” Now, there are lots of chemicals that can contribute electrons and, yes, you guessed it, they are called “reducing agents.” In photography they’re also called “developers.” Usually the developers used in photography are organic compounds, but that’s not a critical point.
Now the key to all of this is that a crystal of silver bromide will not be reduced to free silver unless it already has some free silver in it. Remember the latent image? The free silver kind of primes the pump for the reducing agent. There are two fancy phrases used in chemistry for this pump priming process. We say that the free silver catalyzes the production of free silver in those grains were it already exists. Alternatively, we say that the free silver acts as a nucleation site for the production of more free silver.
In any event, the effect of all of this is that those grains which had free silver, that is are part of the latent image, undergo reduction to produce more free silver. In contrast those which had no free silver are unaffected by the developer.
This is great! Right? We now have lots of dense silver grains (see Figure 1) on the parts of the emulsion, which were exposed to light. So can we turn on the lights now and see the image? Absolutely, not. The problem is that there is still lots of unexposed and, therefore, still light sensitive silver bromide in the emulsion. So there are a few more steps in the development process.
First, you need to stop the action of the developer. While this can be done by rinsing in water, when you are looking for good control of the process, it is more typical to use an acid stop bath that stops development in its tracks. This makes a lot of sense, since, by definition, an acid provides lots of positively charged protons ready to steal away any remaining electrons.
Then the remaining silver bromide needs to be removed. This is generally done using sodium thiosulfate, otherwise known as hypo. This solublizes the free silver bromide out of the emulsion by converting it to bromine and silver thiosulfate ions. These can then be safely washed away, and if you do a good job with your washing, the image stays clean for a hundred years or more.
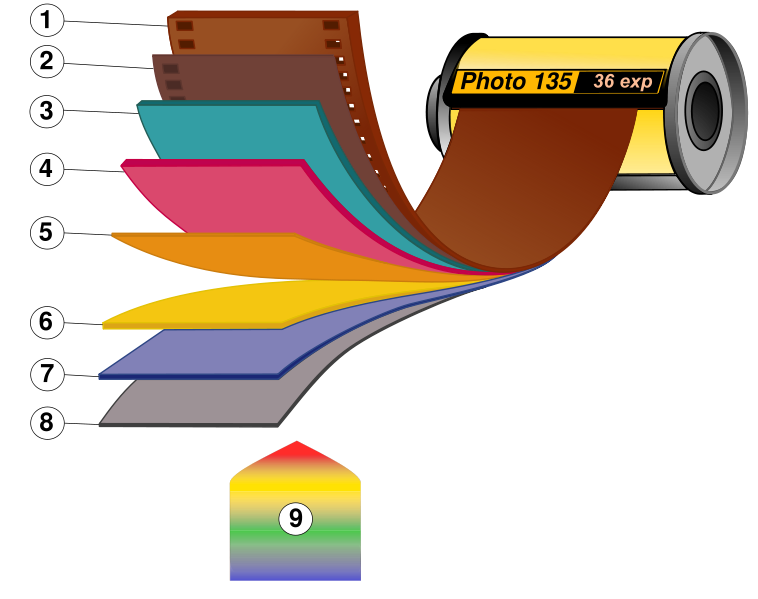
Figure 2 – a modern color photographis film showing the different layers. 1. Film base; 2. Subbing layer; 3. Red light sensitive layer; 4. Green light sensitive layer; 5. Yellow filter; 6. Blue light sensitive layer; 7. UV Filter; 8. Protective layer; 9. Visible light. From the Wikicommons, by Voytek S under creative commons liscense.
This is pretty much all that there is to it. But let’s take a moment to reflect. This brilliant concept was created and refined by hundred of chemists, physicists, and engineers over the course of more than a century. The wonderful complexity is hidden in what seems very simple. Therein lies the magic! The gelatin, for instance, has to allow all the chemistry to occur, allow the removal of unreacted silver bromide, but then offer some reasonable level of mechanical stability for the silver grains for a hundred years or more – and we’re doing this with what? – egg whites and animal bones. It’s really truly marvelous. Figure 2 shows a modern color film with all its complex layering required to produce a subtractive color image as we have discussed previously. And remember, at the root of all of this is the basic silver halide photgraphic process.
